OV888/GV101 capsule & ROCK2 platform
OV888 Posters and Publications »
overview
Ovid entered a collaboration with Graviton Biosciences on May 1, 2023. Together, the companies intend to develop OV888/GV101 and a potential portfolio of selective ROCK2 inhibitors for the treatment of a broad range of neurological conditions with high unmet need.
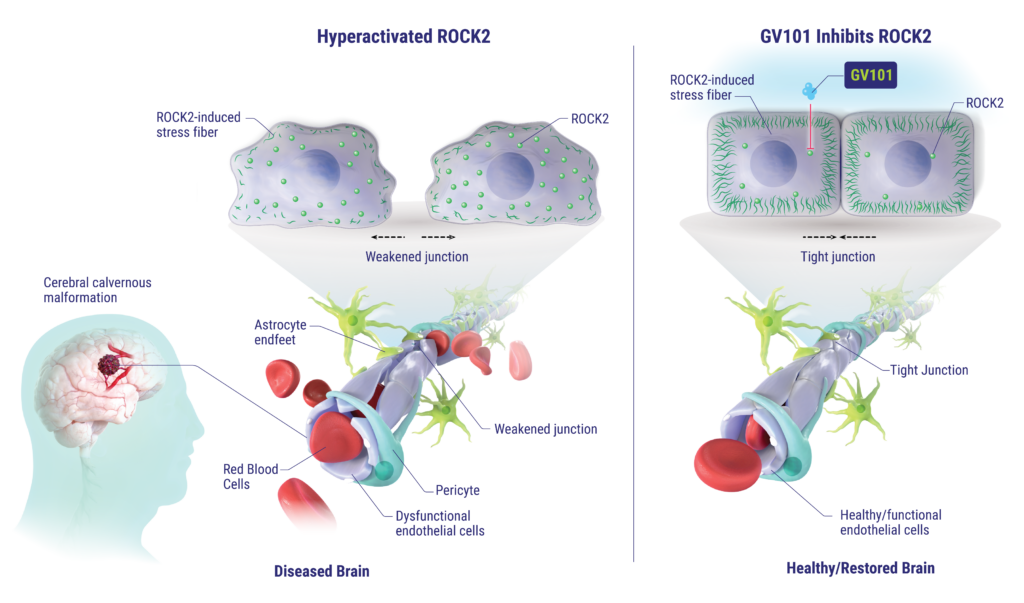
mechanism of action
Rho-associated coiled-coil containing protein kinase 1 and 2 (ROCK1/2) are kinases with isoform differences in tissue distribution. Specifically, ROCK2 is expressed abundantly in skeletal muscles and in the brain and is believed to primarily function to regulate intracellular cytoskeletal organization. Evidence suggests that the ROCK2 signaling pathway may be hyperactivated in multiple neurological diseases, including disorders involving vascular structures and nerve myelination diseases that can result in seizures, spasms and a variety of symptoms. Despite this link, there has been limited clinical development of ROCK2 inhibitors due to challenges penetrating the blood-brain barrier (BBB) and the inherent challenge of avoiding inhibition of ROCK1, which can drive unwanted side effects.
development
OV888/GV101 capsule is a highly selective and potent ROCK2 inhibitor. OV888/GV101 capsule has completed a Phase 1 healthy volunteer study evaluating the safety, tolerability, and pharmacokinetic (PK) profile of multiple ascending doses of OV888/GV101 capsule. The Phase 1 study met its objective, demonstrating a favorable safety and tolerability profile with no serious adverse events. Secondary endpoint results indicate that the target pharmacokinetic profile was achieved at the targeted clinical dose, supporting once daily dosing.
Full topline results of the Phase 1 study can be found here.
Ovid and its partners at Graviton Bioscience received regulatory clearance to initiate a Phase 2 program in patients in Israel and were in the process of applying for clearance in other regions. However, following the recent completion of competitor trials in CCM, and after discussions with key stakeholders, the two companies have decided to pause the initiation of the Phase 2 proof-of concept-study of OV888/GV101 capsule for people with CCM. The strategic pause will allow for further evaluation of insights emerging from these competitor Phase 2 programs in CCM.
Ovid and Graviton continue to pursue additional formulations of OV888/ GV101 for undisclosed cerebral vascular conditions with high unmet need.