soticlestat
overview
Soticlestat is a potential first-in-class oral medicine that is intended to reduce seizure susceptibility and improve seizure control for people living with Dravet syndrome (DS). Ovid co-developed soticlestat through Phase 2 and then sold its rights to Takeda. The two Phase 3 trials evaluating efficacy and safety of soticlestat have completed and top line data was reported by Takeda on June 17, 2024, for more information click here. Ovid retains financial interests to soticlestat.
Takeda will engage with regulatory authorities to discuss the totality of the data generated by these studies to determine next steps. Takeda will also plan to present results of both Phase 3 studies in at an upcoming scientific congress.
mechanism of action
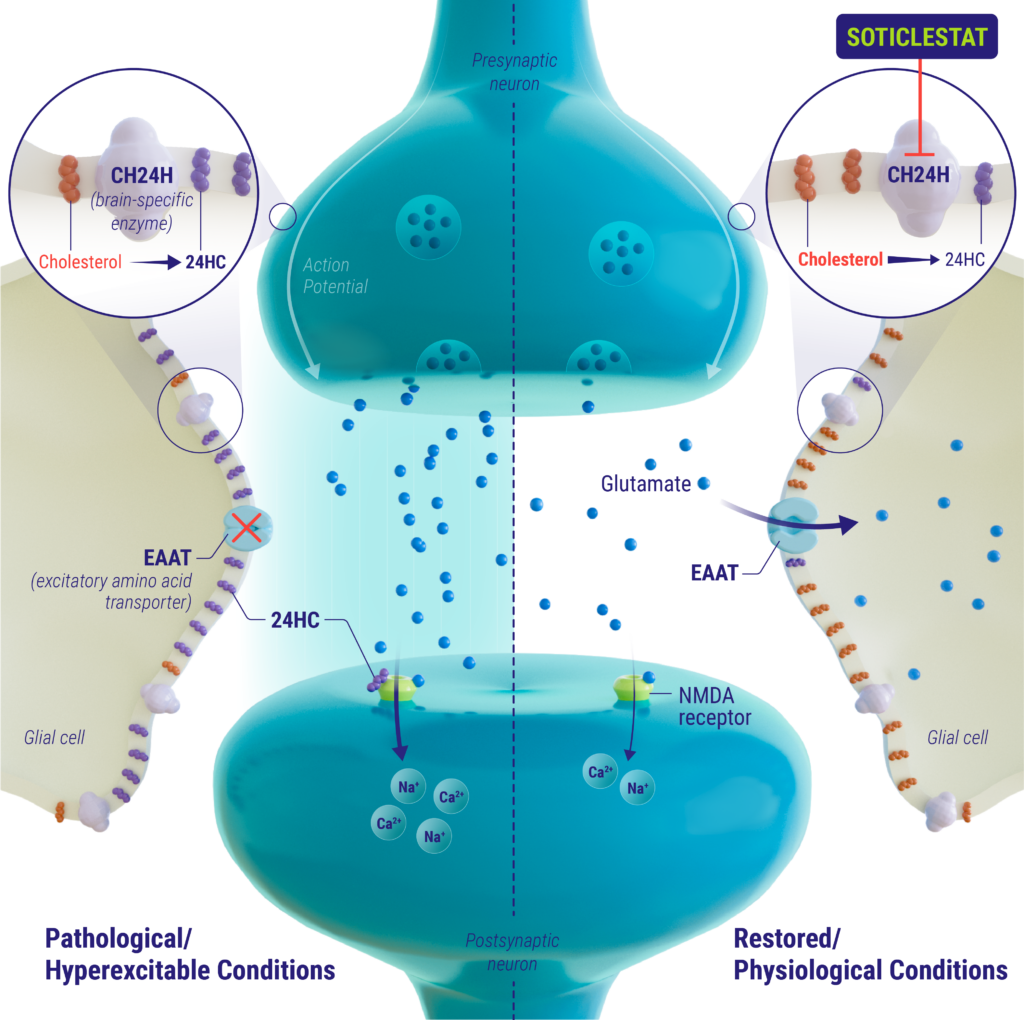
Soticlestat is a potent, highly selective inhibitor of the brain-specific enzyme cholesterol 24-hydroxylase (CH24H). CH24H is predominantly expressed in the brain where it converts cholesterol into 24S-hydroxycholesterol (24HC) to adjust the homeostatic balance of brain cholesterol. 24HC has multiple functions in the brain, one of which is as a positive allosteric modulator of the NMDA receptor. Glutamate is the main excitatory neurotransmitter in the brain and has been shown to play a role in the initiation and spread of seizure activity. Studies suggest that 24HC is involved in over-activation of the glutamatergic pathway through modulation of the NMDA receptor and disruption of astrocytic glutamate amino acid transporter 2 (EAAT2), resulting in epileptogenesis. Inhibition of CH24H by soticlestat reduces the neuronal levels of 24HC and may improve the excitatory/inhibitory balance in the brain, thereby reducing seizure susceptibility and improving seizure control.